The at-home coronavirus test is finally here. Will it change anything?
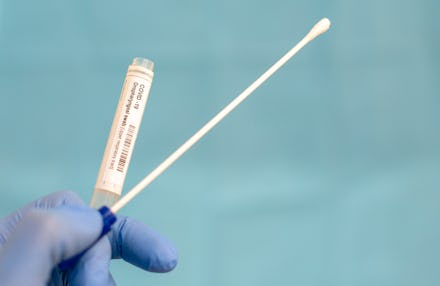
On April 21, the FDA approved a COVID-19 test kit for home use, as in: people can self-sample and send it off to a lab for results. Although about 147,000 tests are completed per day in the U.S., experts think that wider testing is necessary to bring the outbreak under control, reported The Verge. The company that is producing the kit, LabCorp, is giving priority access to the kits will be given to the folks on the front lines — health care workers and first responders — who need it most.
While several companies have tried to create and sell at-home kits, this is the first that the FDA deemed as an equivalent to the kits available in professional labs. “We worked with LabCorp to ensure the data demonstrated from at-home patient sample collection is as safe and accurate as sample collection at a doctor’s office, hospital or other testing site,” said FDA Commissioner Stephen M. Hahn in a public statement. The LabCorp test, like all other COVID-19 tests, has been approved under emergency use rules, meaning that it hasn’t gone through the normal approval process, reported The Verge.
The at-home test, which includes a nasal swab that patients can use on themselves, eliminates the need for a clinician to perform the test. This will effectively lower the amount of health care workers exposed to possible infection. The swab must then be sent to a professional laboratory for testing by way of a sterile pre-paid shipping container, which is included in the $119 price of the test.
This is very good news for the crowds of folks clamoring for at-home testing, and for the rest of us, who will inevitably benefit from more data about who is infected. But it still means that only people who have the means will have more access to testing, making disparities that exist in the U.S. even more obvious.